Digital medical consent management
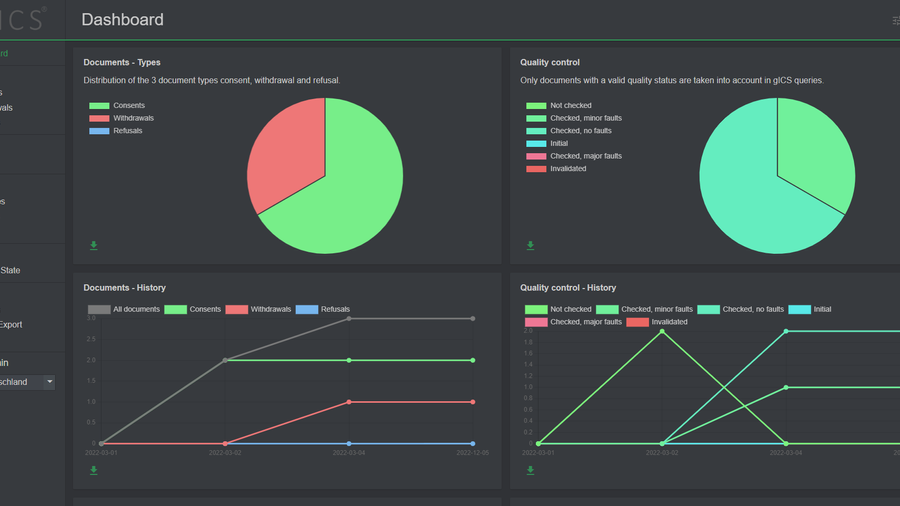
Monitor the health of your clinical trial using clinical consent granting/revocation statistics.
Benefits
Digital technologies almost eliminate the cost of copying data. That provides a lot of small benefits but also a few major ones
Multiple researchers across institutions can collaborate on the same trial.
External and internal auditors can review each patient's data during and after the trial.
You can monitor patient withdrawals as well as calculate early/periodic results.
CRF forms can be imported from a lot of other trials for replication trials; Data is exported in SPSS or CSV formats.
Sending trials details
You need to send us a copy of your trial protocol. It has to include:
Training and testing
You will be contacted to schedule 3 online conferences.
Payment and shipping
You will need to pay after final confirmation that the system meets your needs. Then you will receive a package with hardware. After plugging it in you will enter the same environment you have been testing during the hands-on period.
Research!

Components
Smart trials kit contains many tried and tested tools that are configured to run a completely paperless trial

is the most used clinical research platform. It accepts CRF, handles scheduling, auditing, exports data and does much more. Organizations that use it: WHO; Imperial College London; ESAIC and many more for over 15 years

is a digital consent management platform developed by University Medicine Greifswald. It can both replace paper consent forms or be used in paralel. To know more read our article or try out a demo

is an SPSS alternative. Researchers claim it is more benefit friendly than SPSS. It has the option to use R a popular statical analysis language. You can try it out in the cloud.

Is a pseudonymization tool that creates and maintains a unique set of pseudonyms (for each party or instance dataset is shared). It is developed by University Medicine Greifswald like gICS tool. To know more try out a demo

is a documentation server, that will contain guides and instructions on using all other tools. i.e. How to add another collaborator to data entry or how to use backup a system. It also allows you to write your own documentation on trial procedures and keep secure notes.
A networking system that ensures secure communication allows you to connect to your research data from anywhere in the world. It allows you to move your server any anybody connecting devices regardless of network. If you have an internet connection it should work. For more information read 3rd party security audit summary.

A permission management system that together with local OpenLDAP makes sure that every person participating will need only one set of logins no matter how many tools they are using. It also provides a high level of access customization based on roles or individual users.

Is an industrial firewall that will protect your data from network intrusion no matter where you connect your devices. To learn more you can read a full 3rd party security audit.

together with the "Proxmox Virtual Environment" server, it creates recurring backups of your system to make sure to have a number of copies from different periods of time. Backing up entire virtual systems will allow you to turn back time on your trial or to move it to a different device if were to destroy one.

Is a network firewall hardware provider. You get two devices. The main appliance and a backup one. Protecteli includes hardware security options that will make sure data can not be accessed even if devices are stolen. These devices are also completely silent, small and very power efficient. To know more you can review their warranty policy.
* - On initial contact, you do not need to pay. Payment will be due after final confirmation post remote hands-on time
To start the order please these details in the form below (or ask a question ):
The systems will be tailored to your trial, based on the protocol. The trial protocol should already include what is most significant to proceed: trial schedule; a maximal number of patients; a planned number of visits or consultations; case report form.
It limits how and where data is collected and systems how to be configured to comply with those requirements.
The head researcher\'s contacts will be used in the systems. Also, some of the functionality will be available only to the head researcher due to regulation. If you are contacting on behalf of the research group but you are not the head researcher let us know.
We like to share what technologies are available and how can they be used.

Monitor the health of your clinical trial using clinical consent granting/revocation statistics.

PhD research projects are often small in patient volume and share oversight from same professors
2 minutes... here is how it looks
Is it safe?
Yes. It has multiple layers of protection: All data is sent only over an encrypted connection even in local networks; It includes an industrial firewall; All data on devices is encrypted to prevent access even if the device itself is stolen; Daily backups ensure minimal data loss risk on updates.
Do I need IT support?
No. However, you will need to spend some time adding or removing users who have access to the system but all necessary actions are documented.
Of course, there are exceptions but for additional features. For example, if you want all tools to use the same login and password you use in your institution (LDAP integration support).
Is it GDPR compliant?
Yes. After the moment of purchase, you become the owner of the device. As long as you have the approval of ethics comity to collect data and store it.
We do not have access to your devices and or data.
What clinical trials does it work?
1st or 2nd stage active patient studies or multicenter studies with low participation volumes. Currently, we do not yet offer: SMS/Tele/Online patient surveys; No DICOM image storage; No automated document FHIR data import; Cohort selection from multiple trials.
If any of these features are necessary to you do reach out to let us know what is a priority.
How long does it take from the first email to start using systems?
Hard to say, but 3 months is recommended lead time. It depends on hardware availability, shipping international shipping and the head researcher's time availability.
Can I run more trials on the same system?
It is not recommended. With some additional configuration, you can, however, that will not be covered by warranty. Hardware will be tested to meet the requirements in the clinical trial protocol that you provided.