~ 3 min read
Digital medical consent management
Monitor the health of your clinical trial using clinical consent granting/revocation statistics.
Currier can why can’t doctors?
It is common to have a courier or mailman ask you to sign on a tablet to confirm that you have received mail/package. It may seem strange why the same technology is not utilized for clinical consent gathering. Mail confirmation forms do not change and have no options (i.e. bio bank opt-in), so the same systems can not be used. There is a lot stricter regulation that prevents the centralization of services.
gICS
Facing the same issues researcher group University Medicine Greifswald has developed a digital consent management tool gICS. It meets most problems and is included as part of Smart trials suite.
One problem at the time
Here is a list of problems gICS attempts to solve
Changes
Consent forms may change in the duration of the clinical trial or due to regulation changes.
Consent form versioning allows the creation of multiple versions of the same consent forms. This is handy to keep track of who has not yet updated their consent before it becomes a legal issue.
Options
Clinical research policies may have optional sections that require the patient to select one of the options. GDPR requires that they would not be prefilled by default. Luckily, tables now have touch screens and checking a box is just a poke away.

Digital literacy and Cost
Not every healthcare provider has the option to hand out tables to each patient, and not every patient is comfortable with technology, so gICS has an option to print out a paper form.
Paper forms can then be scanned and uploaded back into the digital system, to have data centralized and keep the statistics. gICS provides a one-click solution to print out the form. QR code is added to the top corner so that when a scan/picture of the paper consent form is uploaded it will automatically add to the correct type of form. Here is a sample of how the printout form looks.
Other benefits
Using digital technologies opens previously unavailable options.
Reuse of policies
The same policy can be used in multiple different consent forms or a new form can be assembled from pieces of other consent forms. This saves a lot of time having to re-approve a new consent by legal. If the said policy is updated it will be updated in all the consent forms using it.
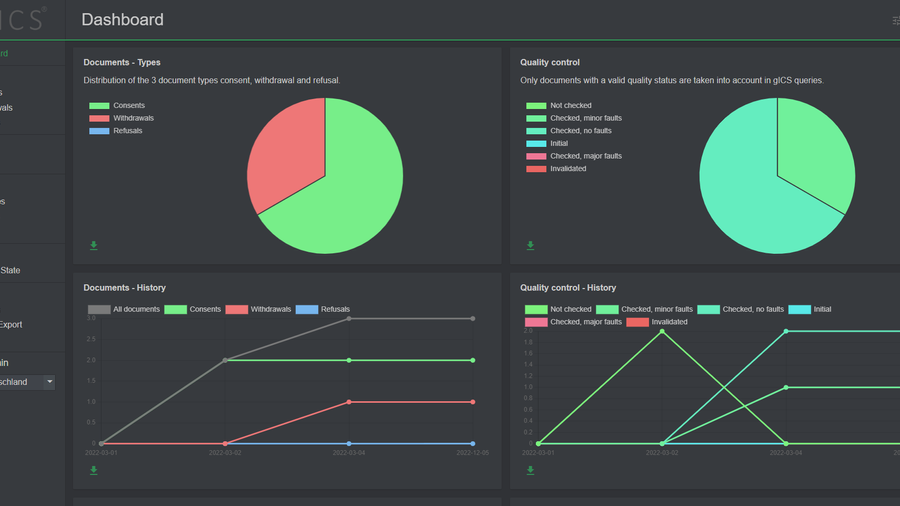
Dashboard
Having an overview of patients that are on trial allows to monitor people quitting or joining the trial. This can
Practice makes perfect
A few tips for the daily use of digital consent forms:
- Keep them docked. Dedicating a place where tablets are collected and immediately connected to charge. Keeping them plugged in most of the time does reduce their lifespan but if the tablet is discharged when consent has to be gathered it can introduce both administrative and legal issues. Stationary kiosks with touchscreen displays are always a good option.
- Paper needs no WiFi. Have a stack of blank forms ready in case of technical problems. Uploading them later may be a little annoying but having to remember to ask the patient to sign next time may be even trickier.
- My name is Department Systems can gather who the person gathering the consent. However, this information is also available in the shift’s schedule, so instead of using filling that field with a name of an individual fill it with the department’s name.
Special sauce
We include a networking solution that will allow a nurse or a doctor to securely collect digital consent from tables that may not be connected to research facility WiFi.
research on gICS
https://dx.doi.org/10.3414/ME14-01-0133
https://dx.doi.org/10.1186/s12967-015-0545-6